Complement in breast milk protects infant mice by shaping gut microbiota
Wan lab assistant scientist Dongqing Xu led research on complement in breast milk that uncovered new roles for the immune system component.
Breast milk provides infants with more than just nutrition; it also includes immune system components, some of them better studied than others.
“Antibodies in breast milk are universally recognized to protect infants against infection and boost immunity,” said Dongqing Xu, PhD, an assistant scientist in the lab of Fengyi Wan, PhD, professor in Biochemistry and Molecular Biology. “As another important part of immune system, the role of ‘complement’ components in breast milk should be explored.”
Xu led research using mouse models that has begun to reveal those roles that was recently published in Cell.

Dongqing Xu, assistant scientist in the Wan lab
“We found the complement in mouse breast milk supports a ‘protective’ gut microbiota in the infant intestine by selectively eliminating specific gram-positive bacterial species,” Xu said.
Further, though complement is named for its roles in working with and “complementing” antibodies, this reshaping activity is independent of antibodies.
“This represents an important expansion of our understanding of breast milk’s protective mechanisms,” said Wan, and reveals a new role of complement proteins, and one that is part of in the non-specific innate immune response.
The Wan lab studies how interactions between host cells, gut microbiota, and pathogens in the colon can affect health and disease, from infections to cancer.
One method they use is a mouse model of transient diarrheal infections, like those caused by certain types of E. coli known as EPEC and EHEC. Mice aren’t susceptible to E. coli the way humans are, but they are susceptible to a similar bacterium called Citrobacter rodientium. As with humans and EHEC and EPEC, healthy mice usually fight off C. rodientium infections quickly.
Researchers in the lab, led by Xu, used mice that had key complement genes knocked out. These “complement-deficient” mice were missing key components of the complement system, and complement-deficient dams (female mice) produce breast milk missing those components.
When weanling (21-day-old) mice pups that still had all the complement genes were given a challenge of C. rodientium, these complement-sufficient mice had minimal symptoms and all survived after the inoculation. But compliment-deficient weanling mice had significant reductions in body weight, severe diarrhea, and over 90% mortality in the same time period. Adult (6-8 week old) mice of all genetic backgrounds were able to resist the infection.
This indicated that complement was important in protecting the infant mice from infections like C. rodientium. But because the mice were nursing from mothers with the same genetic background, this couldn’t separate the effects of the infant’s genetic background from the complement components they received from the breast milk. To separate these, the team developed a new strategy.
“We switched the lactating mothers—complement-sufficient and complement-deficient—immediately upon delivery,” said Xu.
With this cross-fostering, wild-type mice were nursing from complement-deficient dams, and vice versa. And what the team found was striking: pups that nursed from complement-deficient dams, no matter their genetic background, were sensitive to the C. rodientium infections. And pups that nursed from complement-sufficient dams, even pups missing complement genes themselves, resisted the infections.
“We were really thrilled by the cross-fostering results,” said Xu. “The findings from this experiment strongly reinforce our belief that complement components in breast milk are crucial for the health of newborns and infants.”
With a series of experiments, the team found this protective effect was related to the ability of the complement in milk to reshape the composition of microbes in the guts of weanling mice, by eliminating or reducing certain types of microbes.
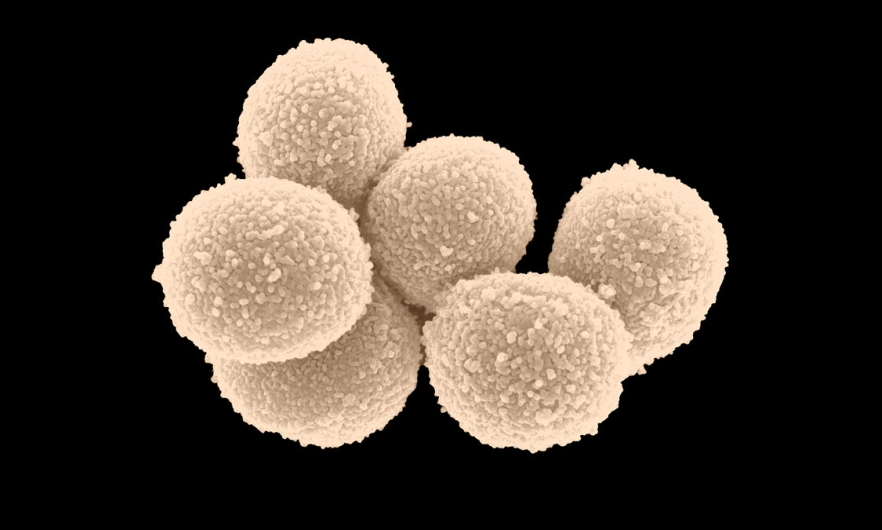
Xu and colleagues isolated one of the strains found in complement-deficient gut microbiota were killed by the complement components of normal milk, and identified it as the gram-positive Staphylococcus lentus B3.
Now that they had a specific target, the team could use in vitro experiments to better understand the components and mechanisms involved. They showed that certain complement components directly targeted and killed S. lentus B3, in human as well as mouse breast milk, and that this activity doesn’t rely on the presence of antibodies.
Xu hopes these finding will spur more work exploring the connection between complement in breast milk on the health of infants, and potential longer-term outcomes.
In the meantime, Xu and the Wan lab are following up these findings and working to better understand the specifics of the complement biology of breast milk and how that differs from much better studied complement biology of blood, such as demonstrating how the breast milk complement kills S. lentus B3 despite past work suggesting that the same components in blood could not kill gram-positive bacteria.
Want to learn more? Check out these commentaries:
Complement(ing) the microbiome in infants through breastmilk, Nobs SP, and Elinav E, Cell Research, 2024.
Complement-ary protection for all ages, Han G, and Vaishnava S, Immunity, 2024.
Complement: you gutsy thing! Kemper C, Trends in Immunology, 2024.
